Research Interests: Chromatin Remodeling, Epigenetic Regulation of Transcription
Background:
The initial event of transcription of any gene requires the formation of a pre-initiation complex which associates with the gene’s promoter region. Once activation of this complex has occurred, the RNA polymerase leaves the promoter region and begins the process of elongation of nascent RNA chains.
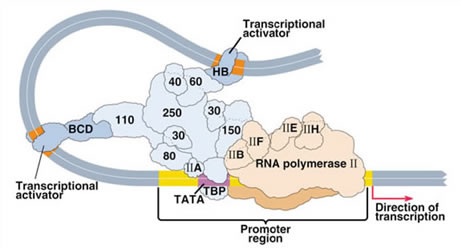
This is the picture that has emerged from countless studies performed with purified transcription factors, purified polymerase and purified DNA as a template. But in cells DNA is not free, it is wrapped around nucleosomes (which are octamers of 4 different histones).

This association is unfavorable to the process of transcription and must be altered in order for the pre-initiation complex to access promoter regions, i.e. in order for transcription to start.
Changes in the association of DNA and nucleosomes are achieved by modifying histones chemically in a manner that alters their affinity for the DNA or simply by shifting the position of nucleosomes along a segment of the DNA molecule. These changes not only make promoters and regulatory regions accessible to the transcription machinery but they also serve as signals to attract specific factors that help in starting the process of transcription.
Once transcription has been initiated, its rate must be regulated so that an appropriate amount of gene product is made. This can be achieved by enhancing re-initiation (the assembly of a new pre-initiation complex after the previous one has left the promoter); it can also be achieved by facilitating the elongation of nascent RNA chains (how fast the polymerase synthesizes RNA) which, in turn, may result in an increase in re‑initiation.
Experimental Approach:
(1) Drosophila: As a model system, we study the mechanism of dosage compensation in Drosophila, a regulatory process that insures that males (with 1 X chromosome) and females (with 2 X chromosomes) have equal amounts of X-chromosome gene products. In Drosophila, this is achieved by doubling the rate of X-linked gene transcription in males, relative to females. Our laboratory has identified five genes involved in this process and we have shown that their protein products form a complex (the MSL complex) that is present all along the X chromosome in somatic cells of males but not of females.

The complex is very unusual in that it contains one of two types of RNA molecules (discovered by Meller et al.; Amrein and Axel). We have shown that this protein complex is responsible for a specific chemical modification of ther X chromosome chromatin - the acetylation of histone H4 at lysine 16 (discovered by Turner et al.).
Using plasmids and cells in culture, our laboratory has obtained preliminary evidence that the MSL complex doubles the rate of transcription of genes on the male X chromosome by facilitating the elongation of nascent RNA transcripts. The faster polymerase molecules can finish synthesizing a transcript, the faster they can re-initiate the process and make more gene product.
Significance: Our findings provide the first indication that histone acetylation has this type of effect on gene transcription.
(2) Humans: We have demonstrated the presence in humans of a complex (hMSL complex) that includes the homologs of at least four of the proteins of the Drosophila MSL complex described above. The human complex exhibits the same specificity for acetylating lysine 16 on histone H4. Similarly to the Drosophila complex, the human MSL is responsible for the majority of H4-lysine 16 acetylation in the cells. In contrast to the Drosophila complex, the human MSL complex targets all chromosomes.
Significance: A recent study has found that the global loss of H4-lysine 16 acetylation is a very common characteristic of human cancers (reported by Fraga et al.). We can speculate, therefore, that the hMSL complex that we have discovered is a major target of regulation during the process of carcinogenesis.
Current Experimental Goals:
- To understand how a chemical modification of a histone (the acetylation of lysine 16 of histone H4) results in an increase in the rate of elongation by a transcribing RNA polymerase in Drosophila.
- To determine if transcription is affected in the same manner in human cells.
- To identify what types of genes are regulated by the hMSL complex in human cells.
- To understand how the MSL and hMSL complexes interact with the many other complexes that are known to affect the structure and organization (therefore the function) of the genetic material.
|