The bi-directional transport of protein and mRNA that occurs through the nuclear pore complex (NPC) in eukaryotic cells is a highly dynamic but highly regulated
process. Not only must the cell maintain the proper influx of critical components, such as transcription factors, in response to changing extracellular
environments, but it must also maintain the proper efflux of mRNA transcripts for these cellular components to be manufactured in the first place. Interestingly,
the precise mechanisms for these processes of protein and mRNA trafficking are still being uncovered. Our overall goal is to understand the mechanisms by which
proteins and mRNA transcripts are shuttled between the nucleus and the cytoplasm. Our studies using the genetically tractable yeast, Saccharomyces
cerevisiae, have focused on:
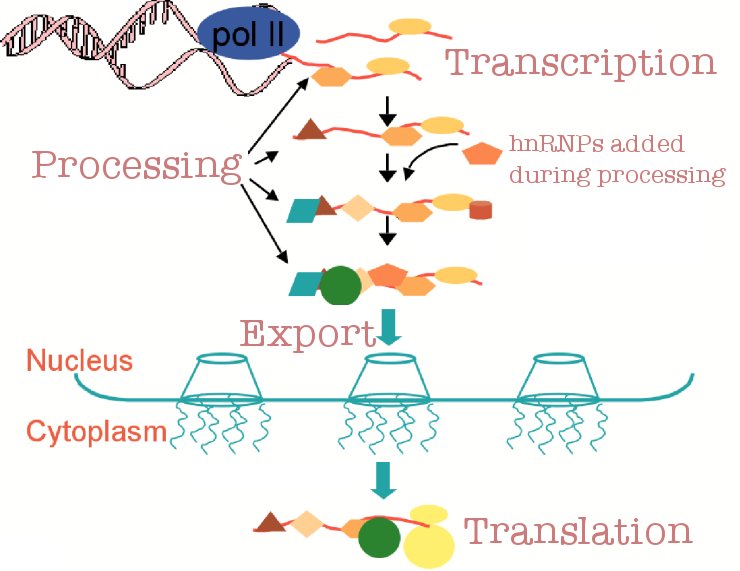
In order for a transcript to be exported from the nucleus, it must first undergo several processing steps including the addition of a 5-methyl-guanosine
cap, splicing out of introns, and addition of a poly(A) tail. In addition, there are also quality control mechanisms to ensure the accuracy of this processing.
These quality control mechanisms are crucial in preventing the release of improperly processed transcripts into the cytoplasm. It is our hypothesis that various
heterogeneous nuclear ribonucleoproteins (hnRNPs) are deposited on transcripts during processing and act as markers to indicate which transcripts have been
properly processed.
In order to more fully understand the role that hnRNPs are playing in mRNA biogenesis and quality control, we have chosen to study the Nuclear Abundant
Poly(A) Binding Protein 2 (Nab2p) as a model for hnRNP function. Our current work focuses on further characterizing interactions between Nab2p and components of
the quality control machinery located at the nuclear pore complex.
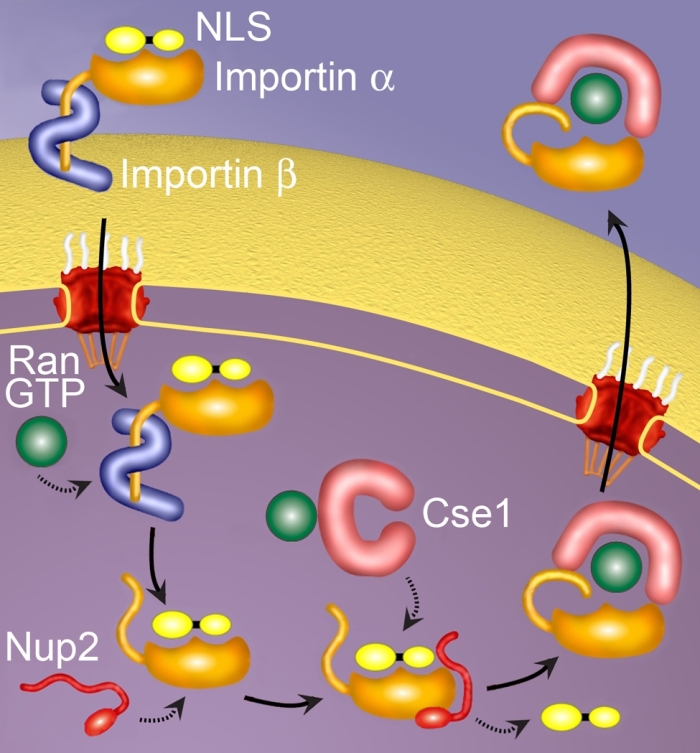
Transport of macromolecules in and out of the nucleus occurs through nuclear pore complexes embedded in the nuclear membrane. In addition, a number of soluble factors are required for nucleocytoplasmic transport. A heterodimeric import complex, importin/karyopherin alpha and beta, recognizes protein cargoes containing classical nuclear localization signals (NLSs) and targets them to the pore. After these complexes are translocated to the interior of the nucleus, they must be disassembled in order to deliver the NLS-cargo. Once dissociated, both importin alpha and beta return to the cytoplasm to participate in subsequent rounds of transport. Three factors have been implicated in NLS-cargo dissociation: an auto-inhibitory function within the N-terminus of importin alpha; the nucleoporin Nup2p; and Cse1p, the protein that recycles importin alpha back to the cytoplasm. Using a combination of biochemical, genetic, and physical studies, we are investigating how these factors specifically affect the dissociation step and working towards a global understanding of how these factors all work together to release NLS-cargo. Thus, our work addresses how soluble factors cooperate with nuclear pore proteins to release NLS-cargo in the nuclear interior.
Lange et al. (2007) JBC Feb 23;282(8):5101-5
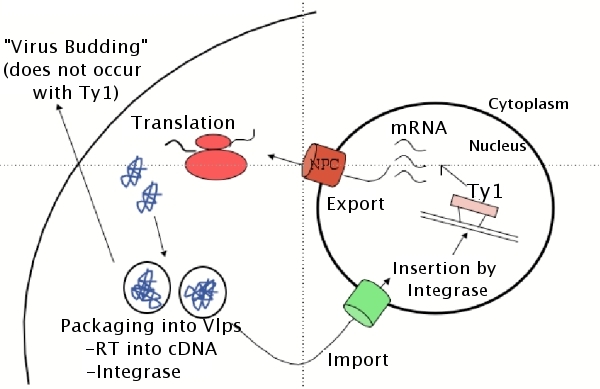
Many retroviruses, like HIV, have developed mechanisms for infecting
non-dividing cells such as macrophages. This suggests that the virus
has the ability to utilize the cells' nuclear transport machinery to
transverse the nuclear membrane and allow access to the chromosomal
DNA for viral integration and subsequent replication. However, the
mechanism used by HIV to cross the membrane barrier has been widely
debated. Because the nuclear membrane does not breakdown during
mitosis in yeast we can use the retrotransposon Ty1, an evolutionary
relative of retroviruses such as HIV, as a model for retroviral transport and
replication. Specifically, we are interested in both the mechanisms
of the nuclear import of the Ty1 cDNA/Integrase complex and the
nuclear export of Ty1 RNA.